The report shows that my country's medical device industry has entered a new stage of high-end development
2019-01-15 09:09:35
The level of medical device manufacturing represents a country's industrial technology level to a certain extent. In the past November, domestic medical devices shined on the international stage. Whether it was the first China International Import Expo held in Shanghai at the beginning of the month or the annual meeting of the Radiological Society of North America (RSNA) in Chicago, USA at the end of the month, the high-profile appearance of domestic large-scale high-end medical devices such as PET-CT and PET-MR, which represent the current cutting-edge technology trends of medical devices, was exciting.
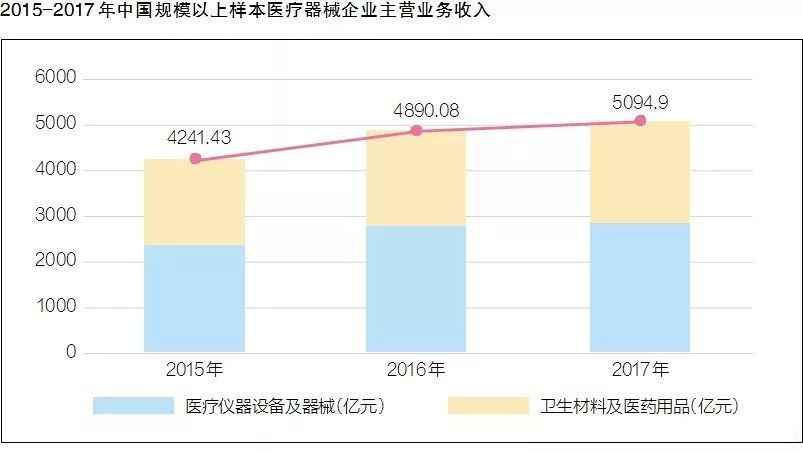
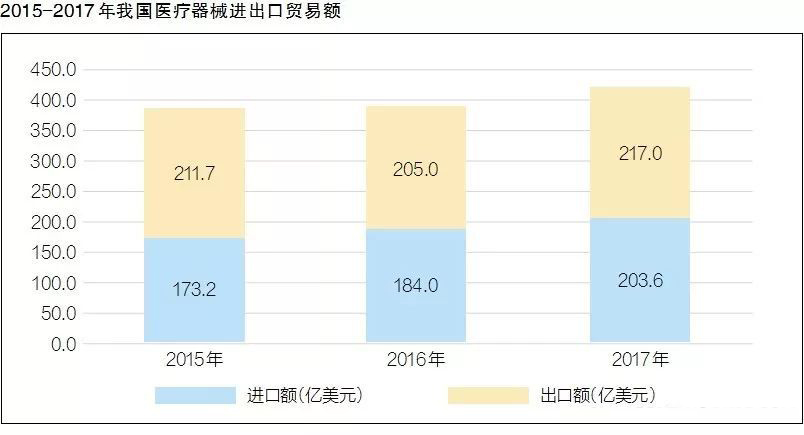
After nearly five years of double-digit annual growth, what are the characteristics of my country's current medical device industry structure? Recently, the China Medical Device Industry Association released the "China Medical Device Industry Development Report 2018" (hereinafter referred to as the "Report"), which analyzed the policy, innovation and other aspects.
Industry status
Overall dispersion Partial concentration
Compared with pharmaceuticals, the medical device industry, which started later, has been in a situation of "many small and scattered" and insufficient innovation for many years. The "Report" pointed out that at present, my country's medical device industry presents a competitive pattern of overall dispersion and partial concentration.
"Many companies are small in scale and cannot be industrialized on a large scale, which makes the product cost high and the profit thin, and further leads to homogeneous competition." A relevant person in charge of the China Medical Device Industry Association said.
According to statistics, in 2014, the output value of 40 large medical device companies in the United States accounted for 20% of the global medical device industry output value, while in the same period, there were about 14,000 medical device manufacturers in China, but the output value accounted for only 13%.
The medical device industry is characterized by diversification, rapid innovation, and difficulty in cross-border operations. It is difficult for enterprises to form a large-scale industry through their own strength. Therefore, mergers and acquisitions are one of the fastest and most effective ways to obtain economies of scale and scope. In addition, the capacity of many medical device market segments is small, and the professional barriers are extremely high. Enterprises cannot achieve rapid growth by relying solely on endogenous growth.
Based on this, the "Report" predicts that medical device companies will accelerate the pace of mergers and reorganizations through various means such as industrial funds, listing financing, and the introduction of foreign capital, continuously improve the level of industry organization, and achieve large-scale and intensive operations. This will be an important trend in the development of the industry in the next few years.
In the three years from 2013 to 2015, there were 45, 69, and 80 medical device mergers and acquisitions in China respectively; there were 2, 8, and 15 transactions of more than US$50 million respectively. This fully demonstrates that mergers and acquisitions in the medical device industry are increasing year by year, and the scale of funds is also increasing. In 2017, there were 84 financing events in the medical device industry, with a cumulative amount of nearly 1.6 billion US dollars.
The report pointed out that after the development in recent years, although a number of representative enterprises such as Mindray Medical, Shanghai United Imaging, Xinhua Medical, Yuyue Medical, Neusoft Group, Lepu Medical, Weigao Group, and MicroPort Medical have emerged in China, most of the production enterprises with annual sales of over 100 million yuan are joint ventures and foreign-funded enterprises. The annual sales of the Japanese medical device market are between 25 billion and 26 billion US dollars, of which there are only more than 60 main enterprises. Although more than 2,000 domestic enterprises have obtained export certification qualifications, they are still mainly small and medium-sized enterprises. In 2016, Lepu Medical's market value exceeded 50 billion yuan, which is still a big gap compared with Johnson & Johnson and Medtronic.
Industrial structure
Create clusters and accelerate innovation
The reporter learned that with the development of my country's medical device industry, some medical device industry clusters and manufacturing development belts have been formed across the country, such as the Pearl River Delta, the Yangtze River Delta and the Beijing-Tianjin-Bohai Bay area, which have become the three major local medical device industry clusters.
According to incomplete statistics, the total output value and sales of medical devices in the three regions account for more than 80% of the national total. Due to different conditions, these three industrial clusters have obvious regional characteristics. In addition, the Chengdu-Chongqing area centered on Chongqing and the Central China area centered on Wuhan are also emerging industrial clusters, featuring biomedical materials, implantable devices and tissue engineering.
On November 25, at the RSNA held in Chicago, a series of products representing cutting-edge medical device technologies competed to debut, among which the PET-MR independently developed by Chinese companies attracted everyone's attention and attracted many professional audiences to share this grand occasion in the "circle of friends".
The "Report" shows that in terms of domestic medical device innovation, my country's medical device industry has basically formed a multidisciplinary cross-research and development system, and has entered a new stage of development from mid-range products to high-end products, from small to large, from weak to strong. The domestic medical device industry is also gradually forming a technology innovation system with enterprises as the main body, market-oriented, and combining production, learning, research and application.
The "Report" analyzes that in the face of highly homogeneous market competition, medical device companies will continue to innovate, speed up product upgrades, and actively explore and develop new products. In terms of policy, the implementation of the special approval procedure for innovative medical devices has accelerated the speed of domestic innovative medical devices to market.
It is understood that since March 2014, the number of products entering the priority review has gradually increased each year. So far, more than 50 innovative products have been approved. In addition, with the advancement of the new medical reform, the income structure of hospitals has changed, and the demand for mid-to-high-end domestic medical devices in hospitals is gradually increasing. Various provinces (autonomous regions and municipalities) have also successively introduced policies and measures to support the development of domestic medical devices. For example, in 2016 and 2017, Sichuan, Zhejiang, Hubei, Guangdong, Anhui, Hebei, Fujian, Liaoning and other provinces have clearly encouraged the use of domestic medical equipment.
Industry goals
Improve standards and align with international standards
On November 28, the official website of the State Food and Drug Administration released the "Key Points and Judgment Principles for Clinical Trial Inspection of Medical Devices", which attracted attention in the industry and everyone rushed to forward and learn.
In fact, since the promulgation and implementation of the revised "Medical Device Supervision and Administration Regulations" in 2014, the corresponding regulations and documents have been released in succession, reflecting the scientific concept of medical device supervision and highlighting the reform and innovation of medical device supervision.
The "Report" pointed out that in order to improve medical device product standards and ensure quality, the country has successively established 24 medical device-related technical standardization committees to be responsible for standard formulation and revision. Since my country became a member of the International Organization for Standardization (ISO) in the 1980s, the international standard adoption rate has been nearly 80%, which has promoted the internationalization of medical device product standards.
Medical device inspection and testing institutions are the technical support units for medical device supervision. According to the analysis of the "Report", there are dozens of medical device inspection and testing institutions recognized by the state, which can basically meet the needs of medical device testing and supervision and management in my country. In November 2015, the former State Food and Drug Administration organized the formulation of the "Qualification Accreditation Conditions for Medical Device Inspection Institutions", which further strengthened the management of medical device inspection institutions and standardized the qualification accreditation of medical device inspection institutions. The promulgation and implementation of this series of measures have improved the supervision and management of medical devices in my country and accelerated its integration with international standards.



